Documentation
Pulsar Instructions

Evolution Instructions

Ultimate Instructions
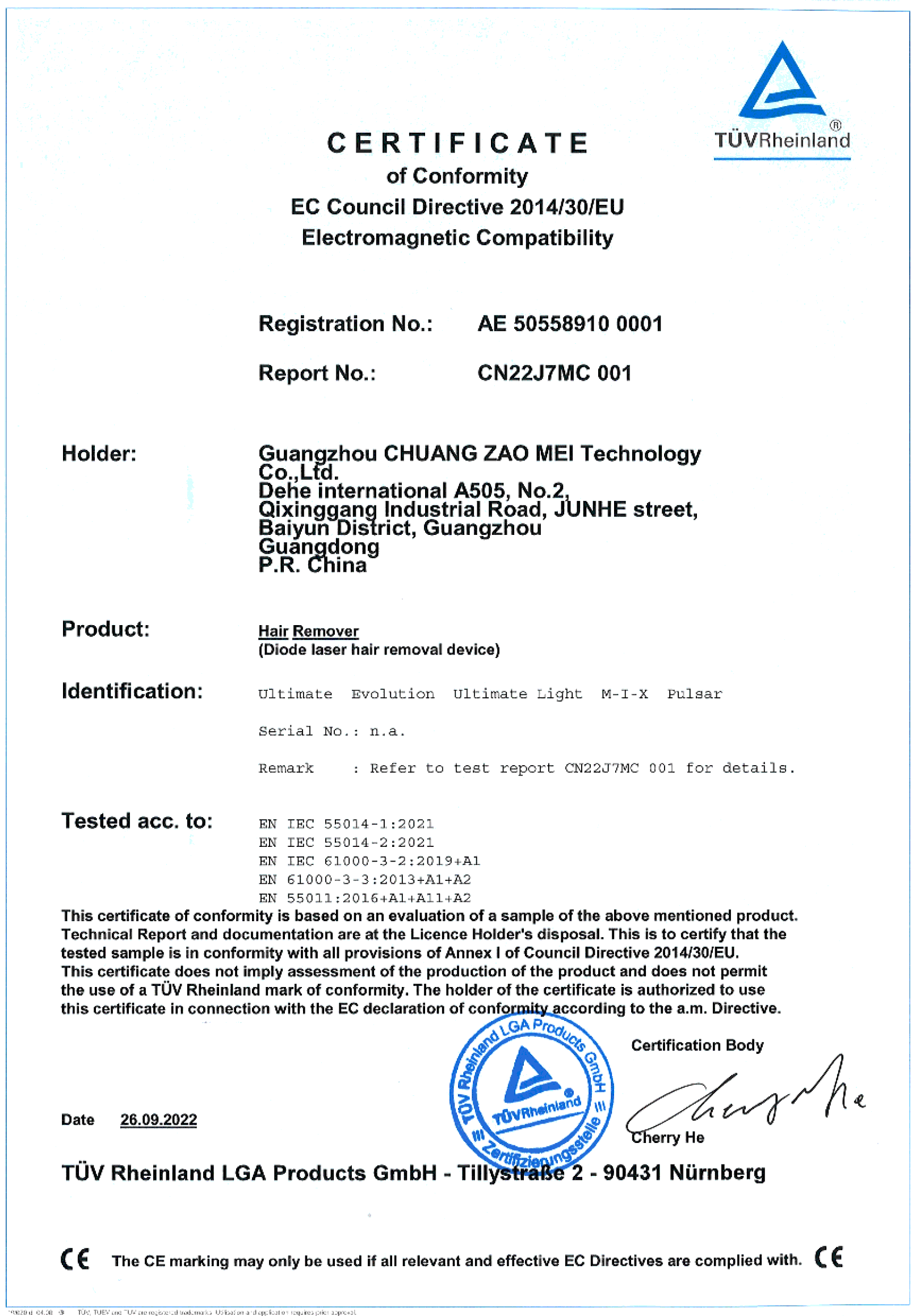
TÜV (short for
from the German Technischer Überwachungsverein)
from the German Technischer Überwachungsverein)
A group of German and internationally recognised expert bodies responsible for technical supervision and monitoring of product safety.
In other words, a test certificate produced by TÜV ensures that the equipment, process solutions and service provided comply with safety standards and quality criteria based on international standards.




If a product is granted FDA approval/cleared by the FDA, it enters the market and guarantees that it does not cause any harm to human health.
That's why, with this certification, you can safely say that your equipment is absolutely safe for everyone. And you have an advantage over other companies
We worked hard for a year to earn this seal of approval, and today we can proudly say
ICBTech equipment is FDA approved
That's why, with this certification, you can safely say that your equipment is absolutely safe for everyone. And you have an advantage over other companies
We worked hard for a year to earn this seal of approval, and today we can proudly say
ICBTech equipment is FDA approved
FDA – Food and Drug Administration
In other words, the organization is dedicated to protecting public health by preventing products from entering the market that might be harmful to people's health or lives.

The standard contains requirements for the quality management system of medical device manufacturers. The requirements for the quality management system established in the standard are additional in relation to the technical requirements for products.”
The main feature of the ISO 13485 standard is that it contains high requirements for the safety of manufactured products.
The main feature of the ISO 13485 standard is that it contains high requirements for the safety of manufactured products.
As a result, on December 31, 2020, the company received the ISO 13485:2016 standard.“ISO 13485 is an international industry standard developed by the International Organization for Standardization.
All Ibtech devices have been certified by SGS, an independent Swiss company providing services for independent examination, control, testing and certification of production and products.

In other words, the certificate of conformity guarantees that the equipment, technological solutions and service provided comply with safety standards and quality criteria based on international standards.
ECM - Twenty years of experience in product certification
Certification of products, machines and working equipment, positioning itself as a reference point for international companies in verifying compliance with standards established by EU directives and major international standards.

We have officially confirmed our commitment to innovation and safety
International certification is an important recognition for our company, reinforcing the trust of our partners and customers.
We value our reputation and ensure that our equipment and technological solutions meet safety and quality criteria based on international standards.
We value our reputation and ensure that our equipment and technological solutions meet safety and quality criteria based on international standards.





